Abstract
The immune system has immense potential in cancer therapy as it is individ-
ualized, precision driven and robust, however, it is associated with challenges
of its own that include immune evasion, development of tolerance and a sus-
tained tumor rejection response. Recent FDA approval of several checkpoint
inhibitors, anti-CTLA4, anti-PD-1, has re-invigorated cancer immunology by
demonstrating that tolerance to cancer can be broken to induce a sustained
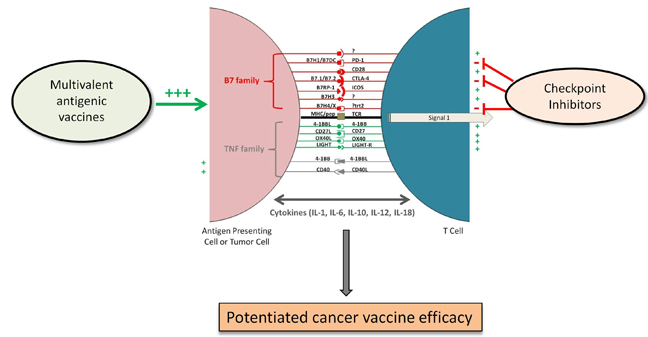
immune response in patients. Active immunization with multivalent tumor
associated antigens (TAA), however, is still a challenge. We have developed
two specific distinct methods to generate multivalent antigens capable of
tumor regression in prostate cancer and melanoma. In prostate cancer, we have
generated specific multivalent peptide mimetics using phage display synthetic
peptide libraries capable of metastatic tumor regression in an animal model. In
melanoma, we have used a vaccinia virus based antigen retrieval technology
to generate a multivalent antigenic vaccine. The antigenic repertoire is well
defined. A protocol for the melanoma vaccine is FDA approved for clinical trials. We envision defining the humoral and cellular immune response to
combine our active vaccine strategy with other treatment modalities including
approved checkpoint inhibitors anti-CTLA4 and anti-PD-1. We believe our
vaccine candidates are a new generation of immune-therapeutics that can
prolong cancer free survival and prevent secondary recurrences. Our studies
have challenged the existing paradigms to re-define cancer immunotherapy
that bridges the gap between humoral and cellular immunity by combining
active immune response with negative checkpoint inhibitors thus activating
pre-existing dormant immune response
References
Baldwin, R. W. (1955). Immunity to methylcholanthrene-induced
tumours in inbred rats following atrophy and regression of the implanted
tumours. Br. J. Cancer 9, 652–657.
Cancer Vaccines: Bench to Bedside 49
Foley, E. J. (1953). Antigenic properties of methylcholanthrene-induced
tumors in mice of the strain of origin. Cancer Res. 13, 835–837.
Prehn, R. T., and Main, J. M. (1957). Immunity to methylcholanthrene-
induced sarcomas. J. Natl. Cancer Inst. 18, 769–778.
Klein, G., Sjogren, H. O., Klein, E., and Hellstrom, K. E. (1960). Demon-
stration of resistance against methylcholanthrene-induced sarcomas.
Cancer Res. 20, 1561–1572.
Greenman, C., Stephens, P., Smith, R., Dalgliesh, G. L., et al. (2007).
Patterns of somatic mutation in human cancer genomes. Nature 446,
–158.
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next
generation. Cell 144, 646–674.
Schumacher, T. N., and Schreiber, R. D. (2015). Neoantigens in cancer
immunotherapy. Science 348, 69–74.
Coulie, P. G., Lehmann, F., Lethé, B., Herman, J., et al. (1995). A mutated
intron sequence codes for an antigenic peptide recognized by cytolytic
T lymphocytes on a human melanoma. Proc. Natl. Acad. Sci. U.S.A. 92,
–7980.
Baldwin, R. W. (1955). Immunity to transplanted tumour: the effect
of tumour extracts on the growth of homologous tumours in rats.
Br. J. Cancer 9, 646–651.
Srivastava, P. (2002). Interaction of heat shock proteins with peptides and
antigen presenting cells: chaperoning of the innate and adaptive immune
responses. Annu. Rev. Immunol. 20, 395–425.
Suto, R., and Srivastava, P. K. (1995). A mechanism for the specific
immunogenicity of heat shock protein-chaperoned peptides. Science 269,
–1588.
Binder, R. J., Han, D. K., and Srivastava, P. K. (2000). CD91: a receptor
for heat shock protein gp96. Nat. Immunol. 1, 151–155.
Schlesinger, M. J. (1990). Heat Shock Proteins: minireview. J. Biol.
Chem. 265, 12111–12114.
Udono, H., and Srivastava, P. K. (1993). Heat shock protein 70-associated
peptides elicit specific cancer immunity. J. Exp. Med. 178, 1391–1396.
Calderwood, S. K., Stevenson, M. A., and Murshid, A. (2012). Heat
shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis.
:486069.
Bunting, S. F., and Nussenzweig, A. (2013). End-joining, translocations
and cancer. Nat. Rev. Cancer 13, 443–454.
N. Tuli et al.
Srivastava, P. K. (2015). Neoepitopes of cancers: looking back, looking
ahead. Cancer Immunol. Res. 3, 969–977.
Suriano, R., Rajoria, S., George, A. L., Geliebter, J., et al. (2013). Ex vivo
derived primary melanoma cells: implications for immunotherapeutic
vaccines. J. Cancer 4, 371–382.
Suriano, R., Rajoria, S., George, A., Jussim, C., et al. (2012). Pro-
ceedings of the 103rd Annual Meeting of the American Association for
Cancer Research: multivalent melanoma vaccine using primary human
melanoma cells and a vaccinia virus based antigen retrieval system.
Cancer Res. 72, 1569.
Wallack, M. K., McNally, K. R., Leftheriotis, E., Seigler, H., et al.
(1986). A Southeastern Cancer Study Group phase I/II trial with vaccinia
melanoma oncolysates. Cancer 57, 649–655.
Wallack, M. K., Meyer, M., Bourgoin, A., Doré, J. F., et al. (1983). A
preliminary trial of vaccinia oncolysates in the treatment of recurrent
melanoma with serologic responses to the treatment. J. Biol. Response
Mod. 2, 586–596.
George, A. L., Suriano, R., Rajoria, S., Osso, M. C., et al. (2015).
PLX4032 mediated melanoma associated antigen potentiation in patient
derived primary melanoma cells. J. Cancer 6, 1320–1330.
Suriano, R., Tuli, N. Y., Geliebter, J., Tiwari, R. K., et al. (2015).
Proceedings of the 106thAnnual Meeting of theAmericanAssociation for
Cancer Research: novel targeted combinational therapies for melanoma.
Cancer Res. 75, 3540.
Larché, M. (2008). Determining MHC restriction of T-cell responses.
Methods Mol. Med. 138, 57–72.
Khanna, R. (1998). Tumour surveillance: missing peptides and MHC
molecules. Immunol. Cell Biol. 76, 20–26.
Nishida, S., Koido, S., Takeda, Y., Homma, S., Komita, H., Takahara,
A., et al. (2014). Wilms tumor gene (WT1) peptide-based cancer vaccine
combined with gemcitabine for patients with advanced pancreatic cancer.
J. Immunother. 37, 105–114.
Mittendorf, E. A., Clifton, G. T., Holmes, J. P., Schneble, E., et al. (2014).
Final report of the phase I/II clinical trial of the E75 (nelipepimut-S)
vaccine with booster inoculations to prevent disease recurrence in high-
risk breast cancer patients. Ann. Oncol. 25, 1735–1742.
Kotsakis, A., Papadimitraki, E., Vetsika, E. K., Aggouraki, D., et al.
(2014). A phase II trial evaluating the clinical and immunologic response
Cancer Vaccines: Bench to Bedside 51
of HLA-A2(+) non-small cell lung cancer patients vaccinated with an
hTERT cryptic peptide. Lung Cancer 86, 59–66.
Jack, A. M., Aydin, N., Montenegro, G., Alam, K., et al. (2007). A
novel dendritic cell-based cancer vaccine produces promising results in a
syngenic CC-36 murine colon adenocarcinoma model. J. Surg. Res. 139,
–169.
Aydin, N., Jack, A., Montenegro, G., Boyes, C., et al. (2006). Expression
of melanoma-associated antigens in human dendritic cells pulsed with an
interleukin-2 gene encoded vaccinia melanoma oncolysate (rIL-2VMO).
Cancer Biol. Ther. 5, 1654–1657.
Suriano, R., Rajoria, S., George, A. L., Geliebter, J., et al. (2013). Follow-
up analysis of a randomized phase III immunotherapeutic clinical trial
on melanoma. Mol. Clin. Oncol. 1, 466–472.
Grotz, T. E., Kottschade, L., Pavey, E. S., Markovic, S. N., et al. (2014).
Adjuvant GM-CSF improves survival in high-risk stage iiic melanoma:
a single-center Study. Am. J. Clin. Oncol. 37, 467–472.
Faries, M. B., Hsueh, E. C., Ye, X., Hoban, M., et al. (2009). Effect
of granulocyte/macrophage colony-stimulating factor on vaccination
with an allogeneic whole-cell melanoma vaccine. Clin. Cancer Res. 15,
–7035.
Slingluff, C. L., Petroni, G. R., Yamshchikov, G. V., Hibbitts, S.,
et al. (2004). Immunologic and clinical outcomes of vaccination with
a multiepitope melanoma peptide vaccine plus low-dose interleukin-2
administered either concurrently or on a delayed schedule. J. Clin. Oncol.
, 4474–4485.
Slingluff, C. L., Petroni, G. R., Olson, W. C., Smolkin, M. E., et al.
(2009). Effect of granulocyte/macrophage colony-stimulating factor on
circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma
vaccine: outcome of a multicenter randomized trial. Clin. Cancer Res.
, 7036–7044.
Overwijk, W. W., Theoret, M. R., and Restifo, N. P. (2000). The future of
interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J. Sci.
Am. 6(Suppl. 1), S76–S80.
Rosenberg, S. A., Lotze, M. T., Yang, J. C., Aebersold, P. M., et al. (1989).
Experience with the use of high-dose interleukin-2 in the treatment of
cancer patients. Ann. Surg. 210, 474–485.
Fifis, T., Gamvrellis, A., Crimeen-Irwin, B., Pietersz, G. A., et al. (2004).
Size-dependent immunogenicity: therapeutic and protective properties
of nano-vaccines against tumors. J. Immunol. 173, 3148–3154.
N. Tuli et al.
Jeanbart, L., Ballester, M., de Titta, A., Corthésy, P., et al. (2014). Enhanc-
ing efficacy of anticancer vaccines by targeted delivery to tumor-draining
lymph nodes. Cancer Immunol. Res. 2, 436–447.
Fang, R. H., Hu, C. M., Luk, B. T., Gao, W., et al. (2014). Cancer
cell membrane-coated nanoparticles for anticancer vaccination and drug
delivery. Nano Lett. 14, 2181–2188.
Liu, H., Moynihan, K. D., Zheng, Y., Szeto, G. L., et al. (2014). Structure-
based programming of lymph-node targeting in molecular vaccines.
Nature 507, 519–522.
Gattinoni, L., Klebanoff, C. A., Palmer, D. C., Wrzesinski, C., et al.
(2005).Acquisition of full effector function in vitro paradoxically impairs
the in vivo antitumor efficacy of adoptively transferred CD8+ T cells.
J. Clin. Invest. 115, 1616–1626.
Gajewski, T. F., Schreiber, H., and Fu, Y.-X. (2013). Innate and adap-
tive immune cells in the tumor microenvironment. Nat. Immunol. 14,
–1022.
Walunas, T. L., Bakker, C. Y., and Bluestone, J. A. (1996). CTLA-4
ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183,
–2550.
McCoy, K. D., and Le Gros, G. (1999). The role of CTLA-4 in the
regulation of T cell immune responses. Immunol. Cell Biol. 77, 1–10.
Jin, H.-T., Ahmed, R., and Okazaki, T. (2011). Role of PD-1 in regulating
T-cell immunity. Curr. Top. Microbiol. Immunol. 350, 17–37.
Wolchok, J. D., Kluger, H., Callahan, M. K., Postow, M. A., et al. (2013).
Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med.
, 122–133.
Hoos, A., Ibrahim, R., Korman, A., Abdallah, K., et al. (2010). Devel-
opment of ipilimumab: contribution to a new paradigm for cancer
immunotherapy. Semin. Oncol. 37, 533–546.
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., et al.
(2010). Improved survival with ipilimumab in patients with metastatic
melanoma. N. Engl. J. Med. 363, 711–723.
Brahmer, J. R., Drake, C. G., Wollner, I., Powderly, J. D., et al. (2010).
Phase I study of single-agent anti-programmed death-1 (MDX-1106) in
refractory solid tumors: safety, clinical activity, pharmacodynamics, and
immunologic correlates. J. Clin. Oncol. 28, 3167–3175.
Azmat, U., Liebner, D., Joehlin-Price, A., Agrawal, A., et al. (2016).
Treatment of ipilimumab induced graves’ disease in a patient with
Cancer Vaccines: Bench to Bedside 53
metastatic melanoma. Case Rep. Endocrinol. 2016, 2087525. doi:
1155/2016/2087525.
Mailleux, M., Cornélis, F., Colin, G., and Baurain, J. F. (2015). Unusual
pulmonary toxicity of ipilimumab treated by macrolides. Acta Clin. Belg.
doi: 10.1179/2295333715Y.0000000047 [Epub ahead of print].
Verschuren, E. C., van den Eertwegh, A. J., Wonders, J., Slangen, R.
M., et al. (2015). Clinical, endoscopic, and histologic characteristics
of ipilimumab-associated colitis. Clin. Gastroenterol. Hepatol. doi:
1016/j.cgh.2015.12.028 [Epub ahead of print].
Horvat, T. Z., Adel, N. G., Dang, T. O., Momtaz, P., et al. (2015). Immune-
related adverse events, need for systemic immunosuppression, and effects
on survival and time to treatment failure in patients with melanoma
treated with ipilimumab at Memorial Sloan Kettering Cancer Center.
J. Clin. Oncol. 33, 3193–3198.
Chaudhuri, D., Suriano, R., Mittelman, A., and Tiwari, R. K. (2009).
Targeting the immune system in cancer. Curr. Pharm. Biotechnol. 10,
–184.
Shanmugam, A., Suriano, R., Goswami, N., Chaudhuri, D., et al. (2011).
Identification of peptide mimotopes of gp96 using single-chain antibody
library. Cell Stress Chaperones 16, 225–234.
Shanmugam, A., Suriano, R., Chaudhuri, D., Rajoria, S., et al. (2011).
Identification of PSA peptide mimotopes using phage display peptide
library. Peptides 32, 1097–1102